
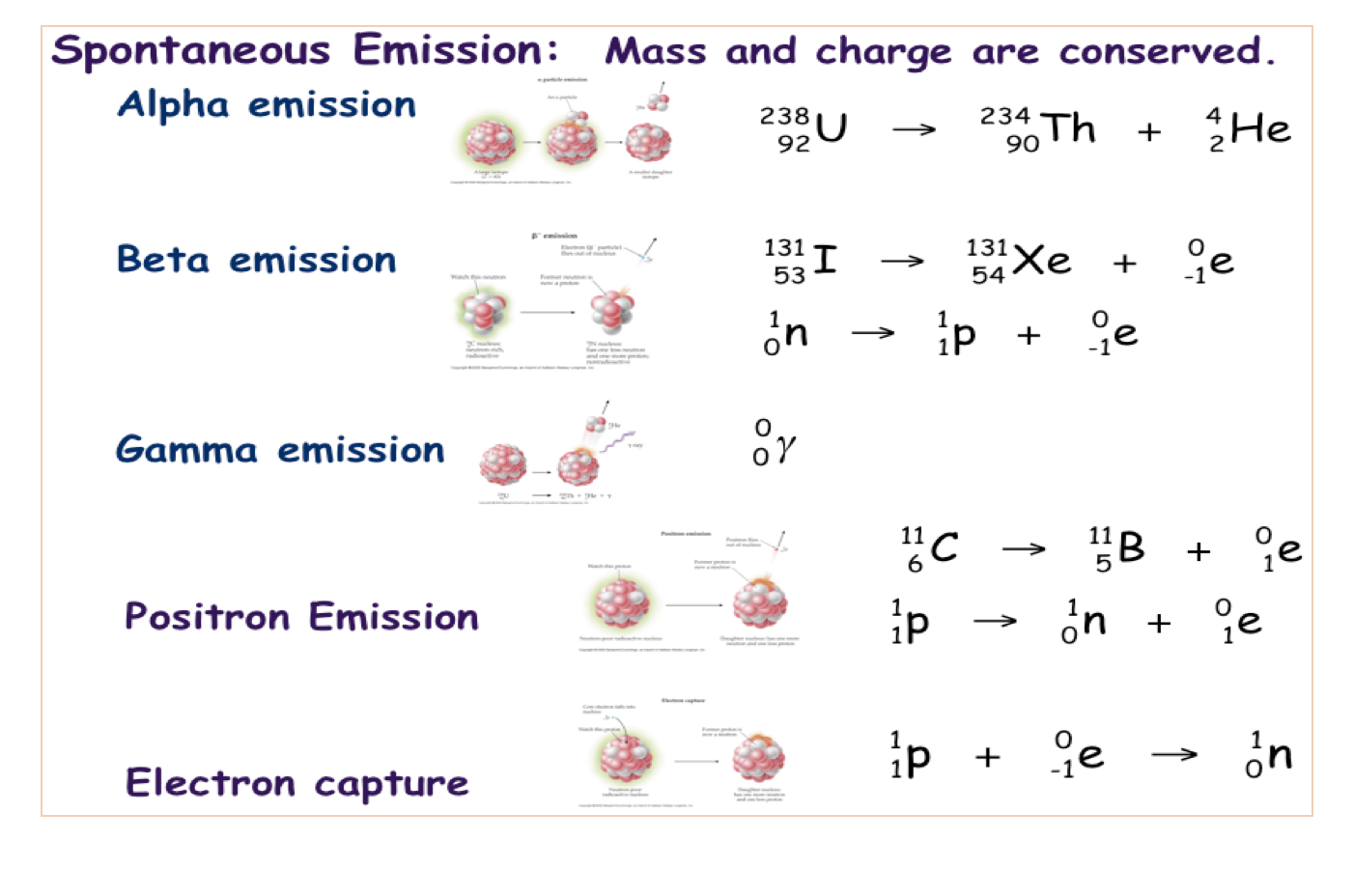
In this reaction, platinum-175 undergoes α-decay to produce osmium-171. The masses of the elements are conserved during alpha decay. parent nucleus and the decay products, which include the daughter nucleus and the particle. In alpha decay, the unstable isotope will emit an alpha particle, along with a more stable isotope (or isotopes). The alpha particle is the same as a helium nucleus with 2 protons and 2 neutrons.
ALPHA DECAY PLUS
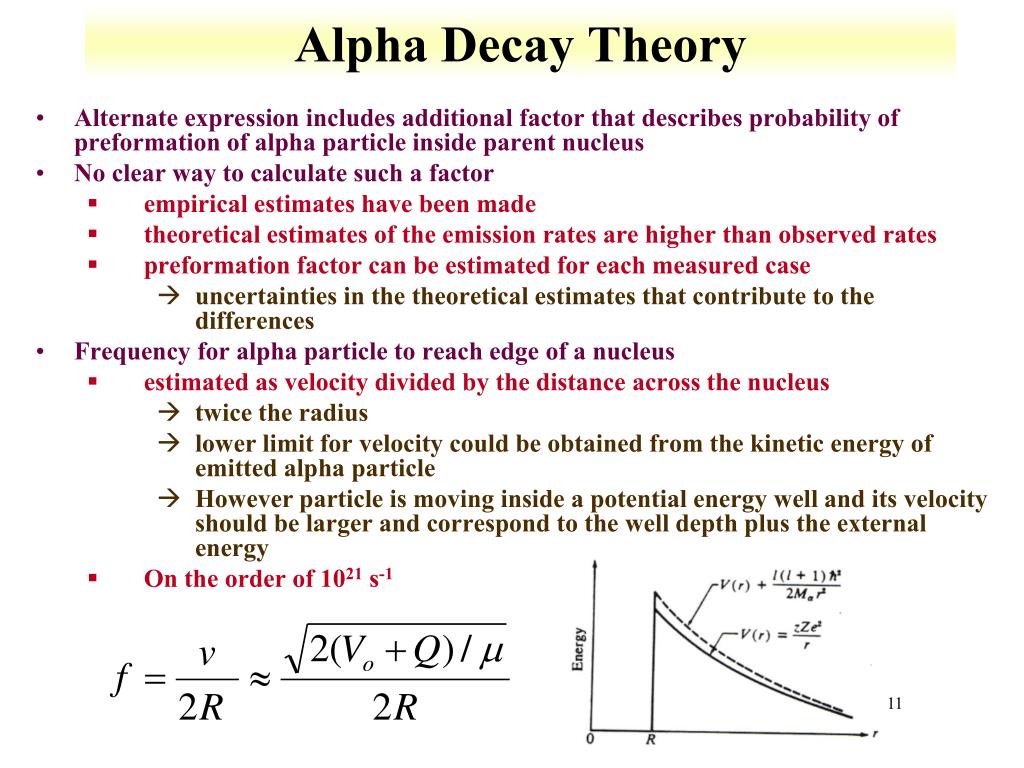
It is identical to a helium nucleus (no electrons). Alpha decay occurs when the nucleus of an atom spontaneously ejects an alpha particle. alpha decay using a simple potential model consisting of the sum of the electrostatic Coulomb potential plus a WoodsSaxon form to represent the alpha-nucleus. Alpha particle: a particle consisting of two protons and two neutrons bound together, identical to a helium nucleus. One of the three main types of radioactive decay is known as alpha decay (α-decay).Īn alpha particle is a name given to a particle that contains two protons and two neutrons. Purpose: We describe alpha decay of 212Po and 104Te by means of the configuration interaction approach. And most of that kinetic energy is in the motion of the alpha particle, particularly if the parent atom was heavy. Background: Alpha emission from a nucleus is a fundamental decay process in which the alpha particle formed inside the nucleus tunnels out through the potential barrier. The isotope splits to create two or more stable particles. The energy release of alpha decay (difference between the rest mass of the initial atom minus the sum of the rest masses of the daughter atom and alpha particle, times c 2) is primarily in the form of kinetic energy. Radioactive decay involves the spontaneous splitting of heavy unstable isotopes.


 0 kommentar(er)
0 kommentar(er)
